ERN ReCONNET
Events & Media
Dissemination materials about ERN ReCONNET and rCTDs.
Dissemination materials developed by ERN ReCONNET and European Commission

ERN ReCONNET Leaflet: click here to download

ERN ReCONNET Clinical Patient Management System Leaflet: click here to download
ERN ReCONNET shares patient stories collected through the JARDIN Joint Action
During Rare Disease Month, ERN ReCONNET is pleased to highlight three powerful patient stories collected within the framework of the JARDIN Joint Action. These testimonies give voice to people living with rare and complex connective tissue and musculoskeletal diseases and underline the importance of coordinated, expert care across Europe. Through their personal journeys, Coralie, Olgaand Lotta,…...New Booklet on ERNs published by the European Commission
Just a few days ahead of the 2025 Rare Disease Day and the European Commission has recently published a series of documents on Rare Diseases, including a new Booklet "European Reference Networks. A success story for patients living with a rare disease". Find out more about ERN ReCONNET and other ERNs at this link: https://lnkd.in/dDeVvd9g…...ERN ReCONNET Awareness Day Campaigns
Here is a collection of the dedicate ERN ReCONNET dissemination slides developed and shared on our social media channels (LinkedIn, X, YouTube, Instagram, @Threads, and Facebook) during the selected awareness days/month. ...Newborn Screening (NBS) Awareness Month
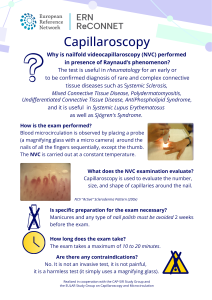
In the US, during 1950s-1960s Dr. Robert Guthrie developed a paper blood spot card test as a new assay to screen newborns for phenylketonuria, PKU: The Newborn Screening (NBS) test was born. His pioneer work defined the beginning of the NBS programs that led to early diagnosis of children with genetic conditions (like PKU, a…...ERN ReCONNET Infographic on Videocapillaroscopy
The ERN ReCONNET Education and Training Working Group (WG) is glad to release its new flyer on nailfold videocapillaroscopy (NVC), a new and informative document targeting patients and lay audience aimed at explaining such an important diagnostic approach. The document was developed by the Network under the leadership of Prof. Maurizio Cutolo from IRCSS –…...ERN: Clinical Practice Guidelines And Clinical Decision Support Tools
There are a number of challenges surrounding the development of Clinical Practice Guidelines (CPGs) and Clinical Decision Support Tools (CDSTs) for rare diseases. One of the most relevant barriers is the lack of high-quality evidence, which cutting-edge methodological frameworks like GRADE rely on. Therefore, there is a need for specific methodological approaches that can provide…...