ERN ReCONNET
Disease Info
Disease: Systemic Sclerosis
Systemic Sclerosis (SSc)
What is SSc?
Clinical overview of the disease
Systemic sclerosis (SSc) is an orphan disease systemic connective tissue disease characterised by autoimmunity, fibrosis of the skin, and internal organs and vasculopathy. Unfortunately SSc has still the highest mortality among all rheumatic diseases especially in patients with lung involvement. Clinical manifestations of SSc can be extremely heterogeneous and this makes it hard to correctly predict its course especially for what concerns internal organ involvement. Clinicians often struggle to diagnose systemic sclerosis early in the disease course. These is often due to late referrals to specialized centres with expertise in managing this complex and rare disease. Moreover, while clinical practical guidelines exist for some specific organ involvements, for others the absence of focused clinical trials prevent from developing strong recommendations. It should also be remembered that SSc is a high burden for patients as it can significantly impair patients’ social and working life and overall their quality of life. This is why the presence of an European network of specialized centres with the involvement of heath-care providers is of fundamental importance.
Epidemiology
The reported prevalence of SSc is 7.2–33.9 and 13.5–44.3 per 100,000 individuals in Europe and North America, respectively. Annual incidence estimates are 0.6–2.3 and 1.4–5.6 per 100,000 individuals in Europe and North America, respectively.
Risk factors and possible causes
Causal factors include several environmental factors alongside genetic susceptibility. Other factors, such as epigenetics, might also be important. Genetic association and sequencing analysis have identified some factors that contribute to genetic susceptibility to SSc and specific complications. There have been reports that associate certain organic chemicals or pesticides with the development of SSc suggesting that exposure to environmental chemicals might be relevant. However, more frequently, chemical exposure has been associated with scleroderma-like diseases that have some characteristics of SSc but distinct clinical phenotypes. These include vinyl chloride monomer and various organic solvents. Noteworthy, some chemotherapy drugs, such as taxanes or gemcitabine, and radiotherapy can trigger SSc or specific complications, which are important considerations in patients who have a concurrent malignancy and SSc. Other chemicals associated with scleroderma-like disorders include L-tryptophan and certain forms of gadolinium, an MRI contrast agent.
Common signs and symptoms
Two major cutaneous subtype of the disease exist according to the extent of the skin fibrotic involvement: limited cutaneous (in case the skin fibrosis is distal to the elbows and knees) and diffuse cutaneous (if there is involvement of proximal skin areas), face is affected in both subtypes. Each cutaneous subtype is associated with a different natural history of the disease, severity of organ involvement, and mortality. Other manifestations that are typical of SSc include the presence of fibrotic changes in the lungs (interstitial lung disease), digital ulcers (which present usually in the fingertips and are a consequence of the vasculopathy typical of the disease), myocardial inflammation and fibrosis and more rarely scleroderma renal crisis (which is an acute kidney injury affecting patients with rapid progressive diffuse cutaneous involvement and which has been associated with exposure of patients to steroids in the early stages of the disease).
Marco Matucci Cerinic
Marco Matucci Cerinic
Disease Coordinator
Disease Coordinator
Details and contents on SSc lay version can be found here.
SSc Lay version (downloadable pdf)
Vanessa Smith
Vanessa Smith
Disease Coordinator
Disease Coordinator
The earliest signs of SSc include Raynaud phenomenon (cold-related (triphasic) vasomotoric reaction in the fingers or toes) and fatigue, which are non-specific for SSc and can have several alternative causes. Efforts have been made to identify patients with Raynaud phenomenon who are at greatest risk for the SSc development. Two validated sets of criteria exist to detect this early stage of SSc. In 2001, the first set of criteria for the early diagnosis of SSc, which included Raynaud phenomenon, SSc-specific antibodies, and scleroderma-type changes on nailfold capillaroscopy were proposed. In 2011, very early diagnosis of systemic sclerosis criteria were proposed and consisted of similar criteria with the addition of puffy fingers. Typically, patients with diffuse cutaneous SSc develop their first non-Raynaud phenomenon symptom of SSc within 1–2 years of the onset of Raynaud phenomenon. Some patients with diffuse cutaneous SSc can develop non-Raynaud phenomenon symptoms in parallel to or within weeks of the onset of Raynaud phenomenon. By contrast, patients with limited cutaneous SSc develop their first non-Raynaud-phenomenon symptom between 5 years to 10 years after onset of Raynaud phenomenon.
When to see a doctor?
The attention of a doctor should be sought potentially in any individual experiencing a new-onset Raynaud’s phenomenon. In this case it is appropriate to look for any additional sign that could be the expression of an underlying connective tissue disease such the presence of puffy fingers, teleangiectasias, inflammatory arthralgias, sclerodactyly and so on. It is recommended for all patients to undergo a capillaroscopy (to see whether there changes in keeping with a scleroderma pattern) and to undergo blood tests including at least ANA antibodies and ideally evaluation of SSc-specific antibodies.
Diagnosis (path/ tests / differential diagnosis)
The differential diagnosis of SSc is quite broad as many diseases can have skin manifestations, vascular features, and organ-based complications. In case of skin fibrosis, other causes of skin or subcutaneous fibrosis, as well as other infiltrative skin diseases should be excluded. Other scleroderma-like diseases are an important consideration that might require expert assessment and treatment. Localised forms of scleroderma are usually distinct from SSc but, occasionally, there can be confusion, especially for generalised morphea.
Differential diagnosis of vascular features includes the many other causes of Raynaud’s phenomenon (which can also be a feature of other CTDs including undifferentiated connective tissue disease, mixed connective tissue disease, inflammatory myositis), as well as other peripheral vascular diseases, especially vasculitis. The inflammatory features of systemic sclerosis are included in the differential diagnosis of several other immune-mediated rheumatic diseases, including lupus, arthritis, and myositis. It should be remembered that not infrequently SSc can overlap with other connective tissue diseases (such as myositis, Sjogren’s syndrome or SLE) and rheumatoid arthritis, and diagnosis of connective tissue disease does not exclude the concurrent diagnosis of SSc. Capillaroscopy and ANA assessment can differentiate between cases of isolated or primary Raynaud’s phenomenon, which represents a common potential differential diagnosis for early SSc.
Treatments overview
Two major strategies are available for the treatment of SSc: Immunosuppressive/anti-fibrotic drugs and vasoactive drugs. Within the European Reference Networks, like in other rare diseases, management of SSc is discussed in multidisciplinary teams. The choice on who deserves treatment and which strategy should be preferred relies on a multiparametric evaluation and goes behind the scope on this summary. We therefore advise patients to discuss their treatments with their physicians.
– Cyclophosphamide is a type of nitrogen mustard drug that when administered in lower dosages modulates regulatory T cells, leading to the decreased secretion of interferon γ and IL-12. This drug was studied in a randomised controlled trial (RCT) in patients with SSc-ILD which demonstrated that 12 months of cyclophosphamide was associated with significant treatment benefits with respect to FVC%-predicted, radiographic fibrosis, health related quality of life and cutaneous fibrosis compared to placebo.
ERN ReCONNET Infographic on Videocapillaroscopy
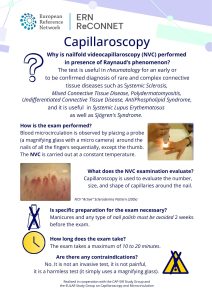 The ERN ReCONNET Education and Training Working Group (WG) is glad to release its new flyer on nailfold videocapillaroscopy (NVC), a new and informative document targeting patients and lay audience aimed at explaining such an important diagnostic approach.
The ERN ReCONNET Education and Training Working Group (WG) is glad to release its new flyer on nailfold videocapillaroscopy (NVC), a new and informative document targeting patients and lay audience aimed at explaining such an important diagnostic approach.
Regarding the NVC approach, the test is mainly useful in rheumatology and in internal medicine, for an early or to-be-confirmed diagnosis of rare and complex connective tissue diseases such as System Sclerosis (SSc), Polydermatomyositis, Mixed Connective Tissue Disease (MCTD), Undifferentiated Connective Tissue Disease (UCTD), AntiPhospholipid Syndrome (APS), and offers important information on microcirculation in Systemic Lupus Erythematosus (SLE) as well as in Sjögren’s Syndrome (SS) and overlaps.
Adverse events (eg, leukopenia, neutropenia, and haematuria) can be common with this treatment this is why, while the evidence was a high-quality study cyclophosphamide is still administered (more commonly intravenously) today for the treatment of dcSSc and SSc-ILD patients in regions with limited access to mycophenolate mofetil (MMF).
– MMF is a prodrug of mycophenolic acid, an inhibitor of inosine monophosphate dehydrogenase which impairs both T cell and B cell proliferation. In Scleroderma Lung Study (SLS) 2 MMF for 24 months was compared to oral cyclophosphamide for 12 months, followed by 12 months of placebo and it was associated with significant improvements in FVC%-predicted, radiographic fibrosis, self-reported dyspnoea, and the extent of cutaneous sclerosis in patients with SSc-ILD. No between-treatment differences were observed but MMF was better tolerated and with fewer adverse events. On this basis, MMF has emerged as a first-line therapy for SSc-ILD and dcSSc, and MMF has now become the background standard therapy for SSc trials including the SENSCIS trial.
– Tocilizumab is a humanised monoclonal antibody that blocks the IL-6 receptor. IL-6 concentrations are elevated in some patients with systemic sclerosis and correlate with the extent of skin involvement. The phase 2 and phase 3 trials with tocilizumab was though no significant difference in mRSS at 48 weeks (primary endpoint) between patients treated with tocilizumab monotherapy and those with placebo. Nonetheless, a significant difference in the change from baseline in FVC%-predicted at 48 weeks, favouring tocilizumab was observed and a post hoc analysis of SSc-ILD patients showed that tocilizumab was associated with stabilisation of FVC%-predicted. The results of the trial suggest that tocilizumab might be effective in SSc-ILD patients with early dcSSc inflammatory features.
– Rituximab is an anti-CD20 monoclonal antibody that depletes peripheral B cells. A recently published RCT demonstrated that treatment with rituximab led to a significant improvement in mRSS compared with placebo (–6,3 vs + 2,14) at 6 months in SSc patients. Most of the patients in this study (89%) had SSc-ILD and treatment with rituximab also had a favourable effect on the change in FVC%-predicted at 6 months. Another recent RCT compared rituximab to cyclophosphamide in patients with CTD-ILD and it showed that treatment with rituximab and cyclophosphamide led to similar improvements in FVC and quality of life; however, rituximab was better tolerated than cyclophosphamide. Rituximab should be used cautionally in patients at high risk for COVID-19 complications, because this agent is associated with impaired humoral responses to COVID-19 vaccination.
– Nintedanib inhibits several tyrosine-kinase receptors, including platelet-derived growth factor receptors, fibroblast growth factor receptors, and vascular endothelial growth-factor receptors. In a large RCT for SSc-ILD, nintedanib slowed the rate of decline of FVC over 52 weeks compared with placebo. Of note, half of the patients were receiving MMF as background therapy. Patients receiving both MMF and nintedanib experienced the slowest decline in lung function. Unfortunately, nintedanib was not associated with improvements in skin score. The majority of patients (76%) on nintedanib had diarrhoea.
– Haematopoietic stem cell transplantation (HSCT) may lead to the most profound improvements in both cutaneous sclerosis and ILD. Three major trials have shown the efficacy of this treatment in SSc patients, its use is thought limited by the its high procedure-related costs and life-threatening adverse events. HSCT is generally considered for patients with early diffuse cutaneous systemic sclerosis as a secondline approach when other therapies fail or as a first-line approach in carefully selected patients.
– Vasoactive drugs include a number of molecules that are used for Raynaud’s phenomenon, pulmonary arterial hypertension and digital ulcers. Three major mechanisms are targeted by these drugs with include endothelin antagonists (bosentan, ambrisentan and macitetan); PDE-5 inhibitors (sildenafil and tadalafil) or a soluble gualylate cyclase stimulator (riociguat) and prostaglandin 2 analogues (iv iloprost and epoprostenol or oral Treprostinil) or prostaglandin 2 receptor agonist (selexipag).
Prognosis
The prognosis of SSc patients has dramatically improved over the years but it still remains the most deadly of all CTDs. The prognosis is driven by the specific subset, the autoantibody profile and most importantly by the specific organ involvement. Today patients with lcSSc and with no significant organ involvement have a prognosis which is similar to healthy individuals whereas patients with dcSSc or with lung, myocardial or kidney involvement need to be carefully looked after to prevent irreversible organ damage or life-threatening complications.
ERN RECONNET PUBLICATIONS ARE AVAILABLE FOR CONSULTATION.
References
Talarico R, Marinello D, Palla I, Cannizzo S, Galetti I, Farrington S, Aguilera S, Andersen J, Ceccatelli E, Cornet A, Cutillas G, Esteves M, Frank C, Leite C, Niehaus G, Perez Gomez E, Polfliet K, Sandulescu S, Schriemer R, Barsotti S, Bellando-Randone S, Beretta L, Bernardino V, Boleto G, Bombardieri S, Burmester G, Cavazzana I, Codullo V, Cutolo M, Dalm V, Damian L, Della Rossa A, Doria A, Farhat MM, Fonseca JE, Hachulla E, Houssiau F, Grazia Lazzaroni M, Limper M, Lorenzoni V, Montecucco C, Mosca M, Mouthon L, Müeller-Ladner U, Pha M, Ponte C, Spierings J, Sulli A, Taulaigo AV, Ticciati S, Tincani A, Toplak N, Trieste L, van Hagen PM, van Laar J, Vanthuyne M, Vigone B, de Vries-Bouwstra JK, Zen M, Turchetti G, Smith V, Matucci Cerinic M. Improving organisation to improve care: ERN ReCONNET organisational reference model for systemic sclerosis patients’ care pathway. J Scleroderma Relat Disord. 2024 Oct 7:23971983241269109. doi: 10.1177/23971983241269109. Epub ahead of print. PMID: 39544904; PMCID: PMC11559522.
– PATIENTS
Social/economic costs and health-related quality of life in patients with scleroderma in Europe. López-Bastida J, Linertová R, Oliva-Moreno J, Serrano-Aguilar P, Posada-de-la-Paz M, Kanavos P, et al. Eur J Health Econ. 2016 Apr;17 Suppl 1:109-17
Patients’ Perspectives and Experiences Living with Systemic Sclerosis: A Systematic Review and Thematic Synthesis of Qualitative Studies. Nakayama A, Tunnicliffe DJ, Thakkar V, Singh-Grewal D, O’Neill S, Craig JC, et al. J Rheumatol. 2016 Jul;43(7):1363-75.
Challenges and strategies for coping with scleroderma: implications for a scleroderma-specific self-management program. Milette K, Thombs BD, Maiorino K, Nielson WR, Körner A,
Peláez S. Disabil Rehabil. 2018
Patients’ Perspectives and Experiences Living with Systemic Sclerosis: A Systematic Review and Thematic Synthesis of Qualitative Studies. Nakayama A, Tunnicliffe DJ, Thakkar V, Singh-Grewal D, O’Neill S, Craig JC, Tong A. J Rheumatol. 2016 Jul;43(7):1363-75. doi: 10.3899/jrheum.151309. Epub 2016 May 1. PMID: 27134259
– HEART
Primary heart involvement in systemic sclerosis, from conventional to innovative targeted therapeutic strategies. Ferlito A, Campochiaro C, Tomelleri A, Dagna L, De Luca G. J Scleroderma Relat Disord. 2022 Oct;7(3):179-188. doi: 10.1177/23971983221083772. Epub 2022 Mar 24. PMID: 36211207
Heart Involvement in Systemic Sclerosis: the Role of Magnetic Resonance Imaging. De Luca G, Bombace S, Monti L. Clin Rev Allergy Immunol. 2023 Jun;64(3):343-357. doi: 10.1007/s12016-022-08923-3. Epub 2022 Jan 24. PMID: 35072931
Primary systemic sclerosis heart involvement: A systematic literature review and preliminary data-driven, consensus-based WSF/HFA definition. Bruni C, Buch MH, Furst DE, De Luca G, Djokovic A, Dumitru RB, Giollo A, Polovina M, Steelandt A, Bratis K, Suliman YA, Milinkovic I, Baritussio A, Hasan G, Xintarakou A, Isomura Y, Markousis-Mavrogenis G, Tofani L, Mavrogeni S, Gargani L, Caforio AL, Tschöpe C, Ristic A
– MALIGNANCY
Malignancies in Patients with Anti-RNA Polymerase III Antibodies and Systemic Sclerosis: Analysis of the EULAR Scleroderma Trials and Research Cohort and Possible Recommendations for Screening. Lazzaroni MG, Cavazzana I, Colombo E, Dobrota R, Hernandez J, Hesselstrand R, et al. J Rheumatol. 2017 May;44(5):639-647.
Systemic Sclerosis Association with Malignancy. Lepri G, Catalano M, Bellando-Randone S, Pillozzi S, Giommoni E, Giorgione R, Botteri C, Matucci-Cerinic M, Antonuzzo L, Guiducci S.Clin Rev Allergy Immunol. 2022 Dec;63(3):398-416. doi: 10.1007/s12016-022-08930-4. Epub 2022 Sep 19.PMID: 36121543
– VASCULAR & ULCERS
Defining Skin Ulcers in Systemic Sclerosis: Systematic Literature Review and Proposed World Scleroderma Foundation (WSF) Definition. Suliman YA, Bruni C, Johnson SR, Praino E, Alemam M, Borazan N, et al. J Scleroderma Relat Disord. 2017;2(2)115-120.
Points to consider for skin ulcers in systemic sclerosis. Galluccio F, Allanore Y, Czirjak L, Furst DE, Khanna D, Matucci-Cerinic M. Rheumatology (Oxford). 2017 Sep 1;56(suppl_5):v67-v71.
Points to consider-Raynaud’s phenomenon in systemic sclerosis. Cutolo M, Smith V, Furst DE, Khanna D, Herrick AL. Rheumatology (Oxford). 2017 Sep 1;56(suppl_5):v45-v48
Exploring the patient experience of digital ulcers in systemic sclerosis. Hughes M, Pauling JD. Semin Arthritis Rheum. 2019 Apr;48(5):888-894. doi: 10.1016/j.semarthrit.2018.08.001. Epub 2018 Aug 11.
Systemic pharmacological treatment of digital ulcers in systemic sclerosis: a systematic literature review. Ross L, Maltez N, Hughes M, Schoones JW, Baron M, Chung L, Giuggioli D, Moinzadeh P, Suliman YA, Campochiaro C, Allanore Y, Denton CP, Distler O, Frech T, Furst DE, Khanna D, Krieg T, Kuwana M, Matucci-Cerinic M, Pope J, Alunno A. Rheumatology (Oxford). 2023 Jun 19:kead289. doi: 10.1093/rheumatology/kead289. Online ahead of print. PMID: 37335850
– GASTRO-INTESTINAL
Gastrointestinal Tract Considerations Part I: How Should a Rheumatologist Best Manage Common Upper Gastrointestinal Tract Complaints in Systemic Sclerosis? Quinlivan A, McMahan ZH, Lee EB, Nikpour M. Rheum Dis Clin North Am. 2023 May;49(2):295-318. doi: 10.1016/j.rdc.2023.01.006. Epub 2023 Feb 28. PMID: 37028836
Gastrointestinal Tract Considerations: Part II: How Should a Rheumatologist Best Manage Common Lower Gastrointestinal Tract Complaints in Systemic Sclerosis? Quinlivan A, McMahan ZH, Lee EB, Nikpour M. Rheum Dis Clin North Am. 2023 May;49(2):319-336. doi: 10.1016/j.rdc.2023.01.007. PMID: 37028837
– RENAL
UK Scleroderma Study Group (UKSSG) guidelines on the diagnosis and management of scleroderma renal crisis. Lynch BM, Stern EP, Ong V, Harber M, Burns A, Denton CP. Clin Exp Rheumatol. 2016 Sep-Oct;34 Suppl 100(5):106-109
Renal Disease and Systemic Sclerosis: an Update on Scleroderma Renal Crisis. Cole A, Ong VH, Denton CP. Clin Rev Allergy Immunol. 2023 Jun;64(3):378-391. doi: 10.1007/s12016-022-08945-x. Epub 2022 Jun 1. PMID: 35648373
– PULMONARY ARTERIES HYPERTENSION
Screening for pulmonary arterial hypertension in an unselected prospective systemic sclerosis cohort. Vandecasteele E, Drieghe B, Melsens K, Thevissen K, De Pauw M, Deschepper E, et al. Eur Respir J. 2017 May 11;49(5).
Denton CP, Campochiaro C, Bruni C, Distler O, Iagnocco A, Matucci Cerinic M. COVID–19 and systemic sclerosis: rising to the challenge of a pandemic. J Scleroderma Relat Disord 2021; 6: 58–65.
List of useful references
Talarico R, Marinello D, Palla I, Cannizzo S, Galetti I, Farrington S, Aguilera S, Andersen J, Ceccatelli E, Cornet A, Cutillas G, Esteves M, Frank C, Leite C, Niehaus G, Perez Gomez E, Polfliet K, Sandulescu S, Schriemer R, Barsotti S, Bellando-Randone S, Beretta L, Bernardino V, Boleto G, Bombardieri S, Burmester G, Cavazzana I, Codullo V, Cutolo M, Dalm V, Damian L, Della Rossa A, Doria A, Farhat MM, Fonseca JE, Hachulla E, Houssiau F, Grazia Lazzaroni M, Limper M, Lorenzoni V, Montecucco C, Mosca M, Mouthon L, Müeller-Ladner U, Pha M, Ponte C, Spierings J, Sulli A, Taulaigo AV, Ticciati S, Tincani A, Toplak N, Trieste L, van Hagen PM, van Laar J, Vanthuyne M, Vigone B, de Vries-Bouwstra JK, Zen M, Turchetti G, Smith V, Matucci Cerinic M. Improving organisation to improve care: ERN ReCONNET organisational reference model for systemic sclerosis patients’ care pathway. J Scleroderma Relat Disord. 2024 Oct 7:23971983241269109. doi: 10.1177/23971983241269109. Epub ahead of print. PMID: 39544904; PMCID: PMC11559522.
New directions for patient-centred care in scleroderma: the Scleroderma Patient-centred Intervention Network (SPIN)
Thombs BD, Jewett LR, Assassi S, Baron M, Bartlett SJ, Maia AC, et al.
Clin Exp Rheumatol. 2012 Mar-Apr;30(2 Suppl 71):S23-9
Frequency and impact of symptoms experienced by patients with systemic sclerosis: results from a Canadian National Survey
Bassel M, Hudson M, Taillefer SS, Schieir O, Baron M, Thombs BD.
Rheumatology (Oxford). 2011 Apr;50(4):762-7.
Social/economic costs and health-related quality of life in patients with scleroderma in Europe
López-Bastida J, Linertová R, Oliva-Moreno J, Serrano-Aguilar P, Posada-de-la-Paz M, Kanavos P, et al.
Eur J Health Econ. 2016 Apr;17 Suppl 1:109-17
Patient participation in patient-reported outcome instrument development in systemic sclerosis
Pauling JD, Frech TM, Domsic RT, Hudson M.
Clin Exp Rheumatol. 2017 Sep-Oct;35 Suppl 106(4):184-192.
Patients’ Perspectives and Experiences Living with Systemic Sclerosis: A Systematic Review and Thematic Synthesis of Qualitative Studies
Nakayama A, Tunnicliffe DJ, Thakkar V, Singh-Grewal D, O’Neill S, Craig JC, et al.
J Rheumatol. 2016 Jul;43(7):1363-75.
Challenges and strategies for coping with scleroderma: implications for a scleroderma-specific self-management program
Milette K, Thombs BD, Maiorino K, Nielson WR, Körner A, Peláez S.
Disabil Rehabil. 2018 May 9:1-10.
Thombs BD, Jewett LR, Assassi S, Baron M, Bartlett SJ, Maia AC, et al.
Clin Exp Rheumatol. 2012 Mar-Apr;30(2 Suppl 71):S23-9
Bassel M, Hudson M, Taillefer SS, Schieir O, Baron M, Thombs BD.
Rheumatology (Oxford). 2011 Apr;50(4):762-7.
López-Bastida J, Linertová R, Oliva-Moreno J, Serrano-Aguilar P, Posada-de-la-Paz M, Kanavos P, et al.
Eur J Health Econ. 2016 Apr;17 Suppl 1:109-17
Pauling JD, Frech TM, Domsic RT, Hudson M.
Clin Exp Rheumatol. 2017 Sep-Oct;35 Suppl 106(4):184-192.
Nakayama A, Tunnicliffe DJ, Thakkar V, Singh-Grewal D, O’Neill S, Craig JC, et al.
J Rheumatol. 2016 Jul;43(7):1363-75.
Milette K, Thombs BD, Maiorino K, Nielson WR, Körner A, Peláez S.
Disabil Rehabil. 2018 May 9:1-10.
New directions for patient-centred care in scleroderma: the Scleroderma Patient-centred Intervention Network (SPIN)
Thombs BD, Jewett LR, Assassi S, Baron M, Bartlett SJ, Maia AC, et al.
Clin Exp Rheumatol. 2012 Mar-Apr;30(2 Suppl 71):S23-9
Frequency and impact of symptoms experienced by patients with systemic sclerosis: results from a Canadian National Survey
Bassel M, Hudson M, Taillefer SS, Schieir O, Baron M, Thombs BD.
Rheumatology (Oxford). 2011 Apr;50(4):762-7.
Social/economic costs and health-related quality of life in patients with scleroderma in Europe
López-Bastida J, Linertová R, Oliva-Moreno J, Serrano-Aguilar P, Posada-de-la-Paz M, Kanavos P, et al.
Eur J Health Econ. 2016 Apr;17 Suppl 1:109-17
Patient participation in patient-reported outcome instrument development in systemic sclerosis
Pauling JD, Frech TM, Domsic RT, Hudson M.
Clin Exp Rheumatol. 2017 Sep-Oct;35 Suppl 106(4):184-192.
Patients’ Perspectives and Experiences Living with Systemic Sclerosis: A Systematic Review and Thematic Synthesis of Qualitative Studies
Nakayama A, Tunnicliffe DJ, Thakkar V, Singh-Grewal D, O’Neill S, Craig JC, et al.
J Rheumatol. 2016 Jul;43(7):1363-75.
Challenges and strategies for coping with scleroderma: implications for a scleroderma-specific self-management program
Milette K, Thombs BD, Maiorino K, Nielson WR, Körner A, Peláez S.
Disabil Rehabil. 2018 May 9:1-10.
Thombs BD, Jewett LR, Assassi S, Baron M, Bartlett SJ, Maia AC, et al.
Clin Exp Rheumatol. 2012 Mar-Apr;30(2 Suppl 71):S23-9
Bassel M, Hudson M, Taillefer SS, Schieir O, Baron M, Thombs BD.
Rheumatology (Oxford). 2011 Apr;50(4):762-7.
López-Bastida J, Linertová R, Oliva-Moreno J, Serrano-Aguilar P, Posada-de-la-Paz M, Kanavos P, et al.
Eur J Health Econ. 2016 Apr;17 Suppl 1:109-17
Pauling JD, Frech TM, Domsic RT, Hudson M.
Clin Exp Rheumatol. 2017 Sep-Oct;35 Suppl 106(4):184-192.
Nakayama A, Tunnicliffe DJ, Thakkar V, Singh-Grewal D, O’Neill S, Craig JC, et al.
J Rheumatol. 2016 Jul;43(7):1363-75.
Milette K, Thombs BD, Maiorino K, Nielson WR, Körner A, Peláez S.
Disabil Rehabil. 2018 May 9:1-10.
Consensus best practice pathway of the UK Systemic Sclerosis Study group: management of cardiac disease in systemic sclerosis
Bissell LA, Anderson M, Burgess M, Chakravarty K, Coghlan G, Dumitru RB, et al.
Rheumatology (Oxford). 2017 Jun 1;56(6):912-921.
Cardiovascular magnetic resonance in rheumatology: Current status and recommendations for use
Mavrogeni SI, Kitas GD, Dimitroulas T, Sfikakis PP, Seo P, Gabriel S, et al.
Int J Cardiol. 2016 Aug 15;217:135-48.
Expert consensus for performing right heart catheterisation for suspected pulmonary arterial hypertension in systemic sclerosis: a Delphi consensus study with cluster analysis
Avouac J, Huscher D, Furst DE, Opitz CF, Distler O, Allanore Y, EPOSS group.
Ann Rheum Dis. 2014 Jan;73(1):191-7.
Bissell LA, Anderson M, Burgess M, Chakravarty K, Coghlan G, Dumitru RB, et al.
Rheumatology (Oxford). 2017 Jun 1;56(6):912-921.
Mavrogeni SI, Kitas GD, Dimitroulas T, Sfikakis PP, Seo P, Gabriel S, et al.
Int J Cardiol. 2016 Aug 15;217:135-48.
Avouac J, Huscher D, Furst DE, Opitz CF, Distler O, Allanore Y, EPOSS group.
Ann Rheum Dis. 2014 Jan;73(1):191-7.
Malignancies in Patients with Anti-RNA Polymerase III Antibodies and Systemic Sclerosis: Analysis of the EULAR Scleroderma Trials and Research Cohort and Possible Recommendations for Screening
Lazzaroni MG, Cavazzana I, Colombo E, Dobrota R, Hernandez J, Hesselstrand R, et al.
J Rheumatol. 2017 May;44(5):639-647.
Lazzaroni MG, Cavazzana I, Colombo E, Dobrota R, Hernandez J, Hesselstrand R, et al.
J Rheumatol. 2017 May;44(5):639-647.
Consensus best practice pathway of the UK Scleroderma Study Group: digital vasculopathy in systemic sclerosis
Hughes M, Ong VH, Anderson ME, Hall F, Moinzadeh P, Griffiths B, et al.
Rheumatology (Oxford). 2015 Nov;54(11):2015-24.
Defining Skin Ulcers in Systemic Sclerosis: Systematic Literature Review and Proposed World Scleroderma Foundation (WSF) Definition
Suliman YA, Bruni C, Johnson SR, Praino E, Alemam M, Borazan N, et al.
J Scleroderma Relat Disord. 2017;2(2)115-120.
Digital ulcers in scleroderma: staging, characteristics and sub-setting through observation of 1614 digital lesions
Amanzi L, Braschi F, Fiori G, Galluccio F, Miniati I, Guiducci S, et al.
Rheumatology (Oxford). 2010 Jul;49(7):1374-82.
Points to consider for skin ulcers in systemic sclerosis
Galluccio F, Allanore Y, Czirjak L, Furst DE, Khanna D, Matucci-Cerinic M.
Rheumatology (Oxford). 2017 Sep 1;56(suppl_5):v67-v71.
Points to consider-Raynaud’s phenomenon in systemic sclerosis
Cutolo M, Smith V, Furst DE, Khanna D, Herrick AL.
Rheumatology (Oxford). 2017 Sep 1;56(suppl_5):v45-v48
Hughes M, Ong VH, Anderson ME, Hall F, Moinzadeh P, Griffiths B, et al.
Rheumatology (Oxford). 2015 Nov;54(11):2015-24.
Suliman YA, Bruni C, Johnson SR, Praino E, Alemam M, Borazan N, et al.
J Scleroderma Relat Disord. 2017;2(2)115-120.
Amanzi L, Braschi F, Fiori G, Galluccio F, Miniati I, Guiducci S, et al.
Rheumatology (Oxford). 2010 Jul;49(7):1374-82.
Galluccio F, Allanore Y, Czirjak L, Furst DE, Khanna D, Matucci-Cerinic M.
Rheumatology (Oxford). 2017 Sep 1;56(suppl_5):v67-v71.
Cutolo M, Smith V, Furst DE, Khanna D, Herrick AL.
Rheumatology (Oxford). 2017 Sep 1;56(suppl_5):v45-v48
Recommendations for the care of oral involvement in patients with systemic sclerosis
Alantar A, Cabane J, Hachulla E, Princ G, Ginisty D, Hassin M, et al.
Arthritis Care Res (Hoboken). 2011 Aug;63(8):1126-33.
Screening and therapy for malnutrition and related gastro-intestinal disorders in systemic sclerosis: recommendations of a North American expert panel
Baron M, Bernier P, Côté LF, Delegge MH, Falovitch G, Friedman G, et al.
Clin Exp Rheumatol. 2010 Mar-Apr;28(2 Suppl 58):S42-6.
Consensus best practice pathway of the UK scleroderma study group: gastrointestinal manifestations of systemic sclerosis
Hansi N, Thoua N, Carulli M, Chakravarty K, Lal S, Smyth A, et al.
Clin Exp Rheumatol. 2014 Nov-Dec;32(6 Suppl 86):S-214-21.
Alantar A, Cabane J, Hachulla E, Princ G, Ginisty D, Hassin M, et al.
Arthritis Care Res (Hoboken). 2011 Aug;63(8):1126-33.
Baron M, Bernier P, Côté LF, Delegge MH, Falovitch G, Friedman G, et al.
Clin Exp Rheumatol. 2010 Mar-Apr;28(2 Suppl 58):S42-6.
Hansi N, Thoua N, Carulli M, Chakravarty K, Lal S, Smyth A, et al.
Clin Exp Rheumatol. 2014 Nov-Dec;32(6 Suppl 86):S-214-21.
UK Scleroderma Study Group (UKSSG) guidelines on the diagnosis and management of scleroderma renal crisis
Lynch BM, Stern EP, Ong V, Harber M, Burns A, Denton CP.
Clin Exp Rheumatol. 2016 Sep-Oct;34 Suppl 100(5):106-109
Lynch BM, Stern EP, Ong V, Harber M, Burns A, Denton CP.
Clin Exp Rheumatol. 2016 Sep-Oct;34 Suppl 100(5):106-109
2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT)
Galiè N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, et al.
Eur Heart J. 2016 Jan 1;37(1):67-119.
Recommendations for screening and detection of connective tissue disease-associated pulmonary arterial hypertension
Khanna D, Gladue H, Channick R, Chung L, Distler O, Furst DE, et al.
Arthritis Rheum. 2013 Dec;65(12):3194-201.
Screening for pulmonary arterial hypertension in an unselected prospective systemic sclerosis cohort
Vandecasteele E, Drieghe B, Melsens K, Thevissen K, De Pauw M, Deschepper E, et al.
Eur Respir J. 2017 May 11;49(5).
Galiè N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, et al.
Eur Heart J. 2016 Jan 1;37(1):67-119.
Khanna D, Gladue H, Channick R, Chung L, Distler O, Furst DE, et al.
Arthritis Rheum. 2013 Dec;65(12):3194-201.
Vandecasteele E, Drieghe B, Melsens K, Thevissen K, De Pauw M, Deschepper E, et al.
Eur Respir J. 2017 May 11;49(5).
Management of systemic sclerosis-associated interstitial lung disease
Roofeha D, Jaafara S, Vummidib D, and Khanna D
Curr Opin Rheumatol 2019, 31:241–249
The identification and management of interstitial lung disease in systemic sclerosis: evidence-based European consensus statements
Hoffmann-Vold A-M, Maher TM, Philpot EE, Ashrafzadeh A, Barake R, Barsotti S, et al.
Lancet Rheumatol 2020; 2: e71–83
Roofeha D, Jaafara S, Vummidib D, and Khanna D
Curr Opin Rheumatol 2019, 31:241–249
Hoffmann-Vold A-M, Maher TM, Philpot EE, Ashrafzadeh A, Barake R, Barsotti S, et al.
Lancet Rheumatol 2020; 2: e71–83
European Dermatology Forum S1-guideline on the diagnosis and treatment of sclerosing diseases of the skin, Part 1: localized scleroderma, systemic sclerosis and overlap syndromes
Knobler R, Moinzadeh P, Hunzelmann N, Kreuter A, Cozzio A, Mouthon L, et al.
J Eur Acad Dermatol Venereol. 2017 Sep;31(9):1401-1424
Preliminary criteria for the very early diagnosis of systemic sclerosis: results of a Delphi Consensus Study from EULAR Scleroderma Trials and Research Group
Avouac J, Fransen J, Walker UA, Riccieri V, Smith V, Muller C, et al.
Ann Rheum Dis. 2011 Mar;70(3):476-81.
Scleroderma (systemic sclerosis): classification, subsets and pathogenesis
LeRoy EC, Black C, Fleischmajer R, Jablonska S, Krieg T, Medsger TA Jr, et al.
J Rheumatol. 1988 Feb;15(2):202-5.
2013 classification criteria for systemic sclerosis: an American college of rheumatology/European league against rheumatism collaborative initiative
van den Hoogen F, Khanna D, Fransen J, Johnson SR, Baron M, Tyndall A, et al.
Ann Rheum Dis. 2013 Nov;72(11):1747-55
Diagnostic criteria, severity classification and guidelines of systemic sclerosis
Asano Y, Jinnin M, Kawaguchi Y, Kuwana M, Goto D, Sato S, et al
J Dermatol. 2018 Apr 23.
Reporting items for capillaroscopy in clinical research on musculoskeletal diseases: a systematic review and international Delphi consensus
Ingegnoli F, Herrick AL, Schioppo T, Bartoli F, Ughi N, Pauling JD, et al.
Rheumatology 2020;0:1–9.
Knobler R, Moinzadeh P, Hunzelmann N, Kreuter A, Cozzio A, Mouthon L, et al.
J Eur Acad Dermatol Venereol. 2017 Sep;31(9):1401-1424
Avouac J, Fransen J, Walker UA, Riccieri V, Smith V, Muller C, et al.
Ann Rheum Dis. 2011 Mar;70(3):476-81.
LeRoy EC, Black C, Fleischmajer R, Jablonska S, Krieg T, Medsger TA Jr, et al.
J Rheumatol. 1988 Feb;15(2):202-5.
van den Hoogen F, Khanna D, Fransen J, Johnson SR, Baron M, Tyndall A, et al.
Ann Rheum Dis. 2013 Nov;72(11):1747-55
Asano Y, Jinnin M, Kawaguchi Y, Kuwana M, Goto D, Sato S, et al
J Dermatol. 2018 Apr 23.
Ingegnoli F, Herrick AL, Schioppo T, Bartoli F, Ughi N, Pauling JD, et al.
Rheumatology 2020;0:1–9.
EUSTAR biobanking: recommendations for the collection, storage and distribution of biospecimens in scleroderma research
Beyer C, Distler JH, Allanore Y, Aringer M, Avouac J, Czirják L, et al.
Ann Rheum Dis. 2011 Jul;70(7):1178-82.
Beyer C, Distler JH, Allanore Y, Aringer M, Avouac J, Czirják L, et al.
Ann Rheum Dis. 2011 Jul;70(7):1178-82.
BSR and BHPR guideline for the treatment of systemic sclerosis
Denton CP, Hughes M, Gak N, Vila J, Buch MH, Chakravarty K, et al.
Rheumatology (Oxford). 2016 Oct;55(10):1906-10.
Update of EULAR recommendations for the treatment of systemic sclerosis
Kowal-Bielecka O, Fransen J, Avouac J, Becker M, Kulak A, Allanore Y, et al.
Ann Rheum Dis. 2017 Aug;76(8):1327-1339.
Is there a role for TNFa antagonists in the treatment of SSc? EUSTAR expert consensus development using the Delphi technique
Distler JH, Jordan S, Airo P, Alegre-Sancho JJ, Allanore Y, Balbir Gurman A, et al.
Clin Exp Rheumatol. 2011 Mar-Apr;29(2 Suppl 65):S40-5.
Recommendations for the management and treatment of systemic sclerosis
Sampaio-Barros PD, Zimmermann AF, Müller Cde S, Borges CT, Freire EA, Maretti GB, et al.
Rev Bras Reumatol. 2013 May-Jun;53(3):258-75.
Denton CP, Hughes M, Gak N, Vila J, Buch MH, Chakravarty K, et al.
Rheumatology (Oxford). 2016 Oct;55(10):1906-10.
Kowal-Bielecka O, Fransen J, Avouac J, Becker M, Kulak A, Allanore Y, et al.
Ann Rheum Dis. 2017 Aug;76(8):1327-1339.
Distler JH, Jordan S, Airo P, Alegre-Sancho JJ, Allanore Y, Balbir Gurman A, et al.
Clin Exp Rheumatol. 2011 Mar-Apr;29(2 Suppl 65):S40-5.
Sampaio-Barros PD, Zimmermann AF, Müller Cde S, Borges CT, Freire EA, Maretti GB, et al.
Rev Bras Reumatol. 2013 May-Jun;53(3):258-75.
Cardiopulmonary assessment of patients with systemic sclerosis for hematopoietic stem cell transplantation: recommendations from the European Society for Blood and Marrow Transplantation Autoimmune Diseases Working Party and collaborating partners
Farge D, Burt RK, Oliveira M-C, Mousseaux E, Rovira M, Marjanovic Z, et al.
Bone Marrow Transplant. 2017 Nov;52(11):1495-1503.
Guidelines of the Brazilian society of bone Marrow transplantation on hematopoietic stem cell transplantation as a treatment for the autoimmune diseases systemic sclerosis and multiple sclerosis
Rodrigues MC, Hamerschlak N, de Moraes DA, Simões BP, Rodrigues M, Ribeiro AA, et al.
Rev Bras Hematol Hemoter. 2013;35(2):134-43.
Consensus statement concerning cardiotoxicity occurring during haematopoietic stem cell transplantation in the treatment of autoimmune diseases, with special reference to systemic sclerosis and multiple sclerosis
Saccardi R, Tyndall A, Coghlan G, Denton C, Edan G, Emdin M, et al.
Bone Marrow Transplant. 2004 Nov;34(10):877-81.
Myeloablative Autologous Stem-Cell Transplantation for Severe Scleroderma
Sullivan KM, Goldmuntz EA, Keyes-Elstein L, McSweeney PA, Pinckney A, Welch B, et al.
N Engl J Med. 2018 Jan 4;378(1):35-47.
Autologous Hematopoietic Stem Cell Transplantation vs Intravenous Pulse Cyclophosphamide in Diffuse Cutaneous Systemic Sclerosis. A Randomized Clinical Trial
van Laar JM, Farge D, Sont JK, Naraghi K, Marjanovic Z, Larghero J, et al.
JAMA. 2014 Jun 25;311(24):2490-8.
Farge D, Burt RK, Oliveira M-C, Mousseaux E, Rovira M, Marjanovic Z, et al.
Bone Marrow Transplant. 2017 Nov;52(11):1495-1503.
Rodrigues MC, Hamerschlak N, de Moraes DA, Simões BP, Rodrigues M, Ribeiro AA, et al.
Rev Bras Hematol Hemoter. 2013;35(2):134-43.
Saccardi R, Tyndall A, Coghlan G, Denton C, Edan G, Emdin M, et al.
Bone Marrow Transplant. 2004 Nov;34(10):877-81.
Sullivan KM, Goldmuntz EA, Keyes-Elstein L, McSweeney PA, Pinckney A, Welch B, et al.
N Engl J Med. 2018 Jan 4;378(1):35-47.
van Laar JM, Farge D, Sont JK, Naraghi K, Marjanovic Z, Larghero J, et al.
JAMA. 2014 Jun 25;311(24):2490-8.
Twenty-two points to consider for clinical trials in systemic sclerosis, based on EULAR standards
Khanna D, Furst DE, Allanore Y, Bae S, Bodukam V, Clements PJ, et al.
Rheumatology (Oxford). 2015 Jan;54(1):144-51.
Systemic sclerosis-associated interstitial lung disease-proposed recommendations for future randomized clinical trials
Khanna D, Brown KK, Clements PJ, Elashoff R, Furst DE, Goldin J, et al.
Clin Exp Rheumatol. 2010 Mar-Apr;28(2 Suppl 58):S55-62.
Khanna D, Furst DE, Allanore Y, Bae S, Bodukam V, Clements PJ, et al.
Rheumatology (Oxford). 2015 Jan;54(1):144-51.
Khanna D, Brown KK, Clements PJ, Elashoff R, Furst DE, Goldin J, et al.
Clin Exp Rheumatol. 2010 Mar-Apr;28(2 Suppl 58):S55-62.
Raynaud’s syndrome in children: systematic review and development of recommendations for assessment and monitoring
Pain CE, Constantin T, Toplak N, Moll M, Iking-Konert C, Piotto DP, et al.
Clin Exp Rheumatol. 2016 Sep-Oct;34 Suppl 100(5):200-206.
Pain CE, Constantin T, Toplak N, Moll M, Iking-Konert C, Piotto DP, et al.
Clin Exp Rheumatol. 2016 Sep-Oct;34 Suppl 100(5):200-206.
Systemic sclerosis and the COVID-19 pandemic: World Scleroderma Foundation preliminary advice for patient management
Matucci-Cerinic M, Bruni C, Allanore Y, Clementi M, Dagna L, Damjanov NS et al.
Ann Rheum Dis. 2020;79:724-726.
International Consensus Criteria for the Diagnosis of Raynaud’s Phenomenon
Maverakis E, Patel F, Kronenberg D, Chung L, Fiorentino D, Allanore Y, et al.
J Autoimmun. 2014 Feb-Mar; 0: 60-65.
Matucci-Cerinic M, Bruni C, Allanore Y, Clementi M, Dagna L, Damjanov NS et al.
Ann Rheum Dis. 2020;79:724-726.
International Consensus Criteria for the Diagnosis of Raynaud’s Phenomenon
Maverakis E, Patel F, Kronenberg D, Chung L, Fiorentino D, Allanore Y, et al.
J Autoimmun. 2014 Feb-Mar; 0: 60-65.
SSc centres in ERN ReCONNET
Helsinki University Hospital, Hospital District of Helsinki and Uusimaa, Finland (ReCONNETFIN) (adult and paedriatric)
- AOU Ospedali Riuniti “Umberto I – G.M. Lancisi-G. Salesi”
- Civil Hospital – Brescia
- AOU Careggi, Florence
- IRCCS AOU San Martino – Genoa
- Foundation IRCCS CA’Granda Ospedale Maggiore polyclinic – Milan
- IRCCS Ospedale San Raffaele di Milano
- ASST Centro Specialistico Ortopedico Traumatologico Gaetano Pini (Presidio Pini)
- AOU di Modena
- AO Padua
- Foundation IRCCS Polyclinic San Matteo, Pavia
- AOU Pisan
- AOU Policlinico Umberto I di Roma
- Fondazione Policlinico Tor Vergata Roma
- Azienda Ospedaliera Universitaria Integrata di Verona
Karolinska Universitetssjukhuset (adult and paediatric)