ERN ReCONNET KICK-OFF MEETING REPORT

26th-28th May 2017 | Pisa, Italy
26th May 2017
AULA MAGNA SCUOLA MEDICA, Via Roma 55, Pisa
27th and 28th May
GRAND HOTEL DUOMO PISA Via Santa Maria, 94 – 56126 PISA
ERN ReCONNET KICK-OFF MEETING REPORT
Introduction
The 24 European Reference Networks were formally launched on March 9th 2017, during the 3rd on ERNs that took place in Vilnius, Lithuania. The Conference celebrated the official birth of the ERNs and represented an historical moment for European healthcare. After the conference in Vilnius, the ERN ReCONNET coordinator announced the ERN ReCONNET Kick-off meeting that was hosted in Pisa, Italy on May 26th-28th 2017.
The goals of the ERN ReCONNET meeting were to initiate the project by:
- Presenting the aims/mission and structure of ERN ReCONNET;
- Ensuring the project participants had a good overview of the aims and structure of the project;
- Identifying, clarifying and establishing responsibility for the 7 WPs of first year (2017);
- Approving the ERN ReCONNET Terms of References (ToR).
Friday 26th May 2017, Aula Magna Scuola Medica, Pisa
The first day of the kick-off meeting was dedicated to disseminate the European Reference Networks and ERN ReCONNET to the research community. To this end, a conference was organized in Pisa at the Medical School of the University of Pisa.
Welcome, Marta Mosca – ERN ReCONNET Network Coordinator
The conference was opened by the Coordinator of ReCONNET, Prof. Marta Mosca, who welcomed and thanked everyone for participating and introduced the speakers.
European Dimension of the Erns, Enrique Terol – European Commission DG SANTE
The session continued with a presentation from Dr. Enrique Terol (DG SANTE Directorate B European Commission) who joined the meeting via WebEx. The presentation “EUROPEAN DIMENSION OF THE ERNS” introduced the concept and the main key elements of the European Reference Networks and focused on the major steps of the first ERNs year and on future initiatives and challenges.
Dr. Enrique Terol outlined the milestone of the ERNs history, from the call in 2016 to the actual 24 approved Networks, providing some sources of funding that could be tapped by the Networks such as: 3rd Health Programme, Erasmus Plus, Horizon 2020 and European Structural and Investment Funds.
The project now is in its implementation phase (2017-2018); during this phase the focus will be on the establishment of ERNs and on the initial organisation of the Networks.
The next phase will be Consolidation (2019-2020) with a full service production, continuous monitoring and performance indicators and initial outcome assessment.
Patients Involvement in the ERNs, Lenja Katharina Wiehe (Eurordis)
Lenja Wiehe form EURORDIS-Rare Disease Europe continued with a presentation on “PATIENTS INVOLVEMENT IN THE ERNS”. The European Commission Delegated Decision of 10 March 2014 (2014/286/EU) defined that the ERNs will have to demonstrate a patient-centred approach and empower patients. In order to involve patient representatives to play an active role in the ERNs, EURORDIS has developed a “European Patient Advocacy Groups” (ePAGs) for each Network, following the established thematic grouping.
ePAGs will bring together elected patient representatives (of the diseases included in ERNs) and affiliated organisations who will ensure that the patient voice is heard throughout the ERN development process. As of May 2017, there are over n. 100 ePAG patient representatives to represent the wider patient community in the development of ERNs. ePAG patient representatives have an official permanent mandate to represent ePAG member organisations. They liaise with these organisations to ensure true and equitable representation of the patients’ voice by participating in the Board and sub-clinical committees of their respective ERN.
The main objectives of ePAGs are:
- Ensure care is patient-centred and respects patients’ rights and choice;
- Ensure transparency in quality of care, safety standards, clinical outcomes and treatment options;
- Ensure ethical issues for patients are addressed, balancing patient and clinical needs;
- Contribute to the development of patient information, policy, good practice, care pathways and guidelines;
- Advise on planning, monitoring and evaluation of ERN initiatives.
EURORDIS is also developing an ePAG leadership programme that includes RD ACTION ERN workshops and ePAGs webinars. The programme will empower ePAG patient representatives with the knowledge and skills they need to be able to effectively participate in ERN activities.
ERN ReCONNET Overview, Marta Mosca – ERN ReCONNET Coordinator
Prof. Marta Mosca, ERN ReCONNET Coordinator, after an introduction on ERNs mission and vision, has took participants through the history of ERN ReCONNET, from the initiative of a group of experts that gave the input to create the ERN ReCONNET to the actual Network, involving 26 full members from 8 different EU countries.
In the Figure 1, the main steps that brought ERN ReCONNET to life:
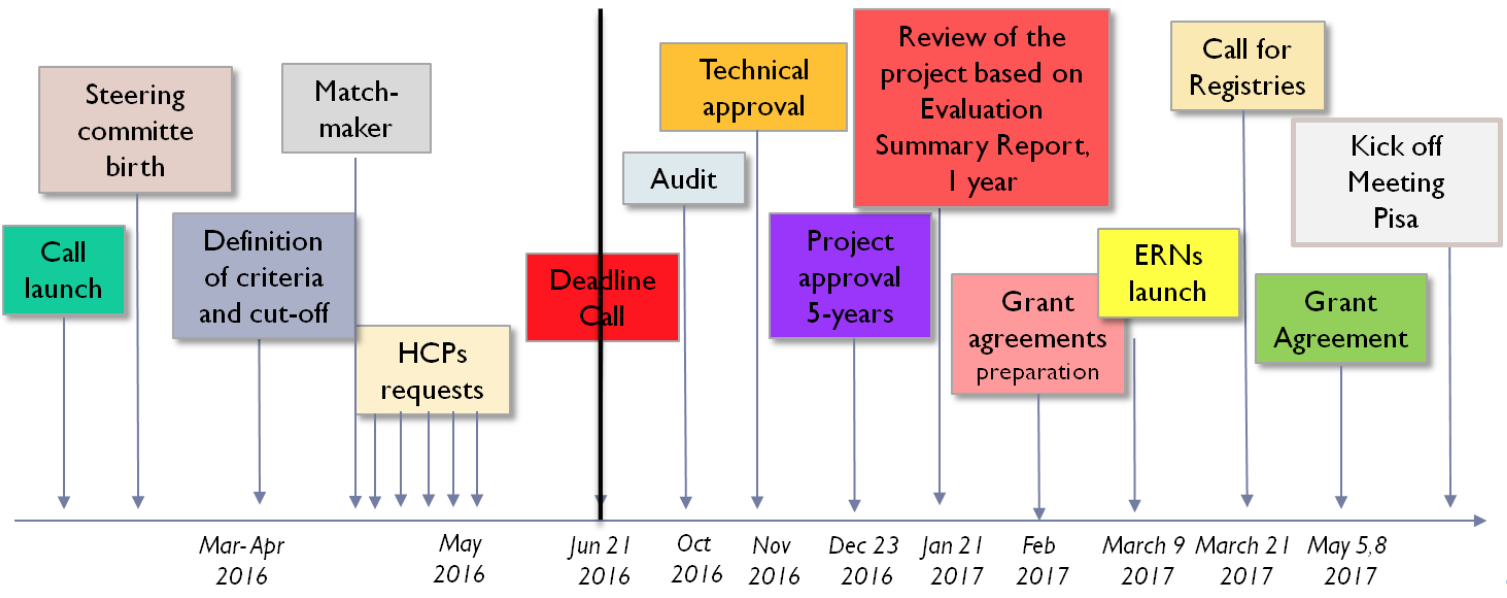
Main steps of ERN ReCONNET
Saturday 27th May 2017, Grand Hotel Duomo, Pisa
Session 1: Overview of Day 2; ERN ReCONNET Terms of Reference Approval; ERN ReCONNET Bodies definition
The first part of this session was dedicated to the mission and vision of the ERN ReCONNET, that include the pursue of cost-effective care, patient-centred approach and the increase of knowledge, education and research in the Rare and complex Connective Tissue and Musculoskeletal Diseases (rCTDs) field.
The ERN ReCONNET Terms of Reference (ToR) has been reviewed by the Network Coordinator and amended following the HCPs proposals. After discussion the ToR was approved in all its parts.
Governance structure of ERN ReCONNET
The Network bodies are:
- the Network Coordinator (NC);
- the Steering Committee (SC);
- The Executive Board (EB);
- the Board of Network (BoN);
- the External Scientific Advisory Board (ESAB).
Their composition and roles were approved.
Professor Maurizio Cutolo was voted as Deputy Coordinator of the ERN ReCONNET.
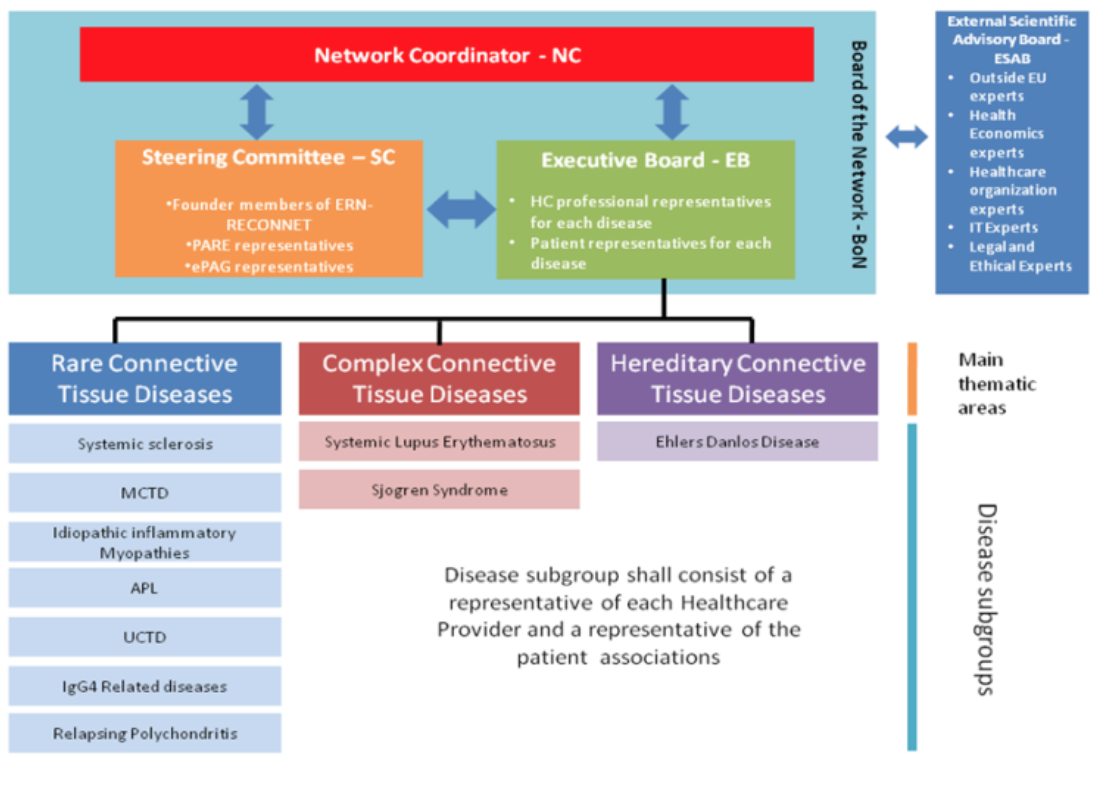
Governance of ERN ReCONNET
The European Commission will launch a call for affiliated members, information and inclusion criteria will be sent to all HCPs who expressed interest in becoming a member of ERN ReCONNET.
Session 2: First year work plan
WP1 – MANAGEMENT AND COORDINATION (M1-M12)
This WP aims at establishing the overall coordination, management and supervision of the Network activities ensuring timely reports and communication between members and with the European Commission/Chafea (EC), administrative and financial management, promotion of stakeholders’ engagement and organization and facilitation of meetings and communication activities. Milestones of this WP will be the approval of the standard procedure manual, election of the bodies’ members and the organization of the open meetings.
WP2 EVALUATION (M3-M12)
This WP aims at identifying to what extent the individual WPs will enable the Network to meet its objectives, to assure that the produced knowledge and outcomes meet high quality standards, are visible and have a relevant impact. Milestones of this WP will be the development of the evaluation plan and ongoing monitoring and evaluation processes and systems with possible adjustments over time (to include publishing, technical support, information gathering, efficiency gains and metrics).
WP3 – DEVELOPMENT OF STANDARD CLINICAL GUIDELINES AND PRACTICE (M4-M12)
This WP refers to the activities aimed at reviewing current practices and guidelines, identifying best practices and standards for the diagnosis and management of the different diseases. This WP provides inputs to WP8 (Education), WP5 (Economic and organizational issues), WP9 (Ethical and legal issues) and it is also related with activities performed in WP10 (Data integration and sharing) and WP6 (Dissemination and Communication). Milestones of the WP will be the review and surveys of guidelines and practice, draft version and final version of standard guidelines and practice.
One of the most important activities for year 1 is the Task 3.1 – In depth revision of the existing guidelines and practice for rCTDs.
To perform this task, one coordinator for each disease was nominated. During the discussion, the identification of junior coordinators was proposed. Junior coordinators are young fellows who have the role to support and cooperate with the senior coordinator in his/her work.
Moreover, it was agreed that the same HCP can appoint only one coordinator for the same disease (either a Senior or a Junior).
It was agreed that members of the Steering Committee will not be appointed as Senior Disease Coordinator, but they can nominate another member of their staff according to the area of expertise.
Both disease coordinators (Senior and Junior) will be responsible for the tasks related to the disease they are representing and will manage and report about the disease they are responsible for.
Senior coordinators are members of the Executive Board (EB). Junior coordinators can attend to the EB meetings.
The NC and the Steering Committee (SC) are responsible for the final review and agreement of all reports.
WP4 – ADAPTATION AND DEFINITION OF THE CONTENTS OF THE CENTRAL ERN IT PLATFORM AND ENVIRONMENT (M6-M12)
The aim of WP4 is to provide an interactive IT platform and IT environment to support ERN-ReCONNET’s activities, implementing and customising the contents of the central ERN IT platform with the specific needs of the ERN-ReCONNET.
WP5 – ECONOMIC AND ORGANIZATIONAL ISSUES (M7-M12)
The aim of this WP is to establish the current burden of rCTDs in terms of resources use and policies, in order to develop a collaborative framework to design and apply standard practices for health economic assessment, the establishment of rules for a proper implementation of HTA for the identification of sustainable and cost-effective pathways for the diagnosis and management of rCTDs across EU centres.
WP5 will benefit from the contribution of Scuola Superiore Sant’Anna, Pisa, and other ESAB members with expertise in economic issues will participate.
WP6 – COMMUNICATION AND DISSEMINATION (M1-M12)
The aims of this WP are to raise the awareness about the outcomes achieved in the different WPs. In this WP all HCPs members and the target groups of ERN ReCONNET are involved in order to promote activities and foster the impact of the ERN activities and results.
Milestones of this WP are the development of the project dissemination plan, and the organization of periodic meetings.
As part of this WP, the HCPs were asked to mention the ERN ReCONNET initiative in each presentation they will give in the future, especially at national level.
A set of slides has been prepared to use for dissemination, together with communication tools developed by the European Commission (see files attached). The HCPs were also asked to provide feedback to the NC every time they will provide information on the ERNs during their presentation.
WP7 – NETWORKING AND SUSTAINABILITY OF ERN (M2-M12)
The aim of this WP is to develop actions in order to create a working team dedicated to the exploration of mechanisms for ensuring networking of the ERN and the long-term sustainability of the Network.
Milestones of this WP will be the survey of all the possible connections, sources of funding, the organization of presentations of the aims and opportunities of the Network to potential funders.
Session 3: IT Collaborative Platform
The final session was dedicated to a practical demonstration of the ERN IT Collaborative Platform from Simone Barsotti (AOUPI – Pisa).
The IT Collaborative Platform supports the ERN Board of Member States, the ERN Coordinators and ERNs members in their on-line communication, document management and event organization.
The platform is not for the exchange of patient data (personal or clinical) and a dedicated tool is being developed by the European Commission and should be ready for July 2017.
Simone Barsotti showed the HCPs how to register and how to use the Platform, providing an overview of the activities to be done with this tool. The patient representative will also have access to the platform and will actively participate.
The platform has a forum with different sections, a library, and an agenda. A user guide is also available for all members.
Sunday 28th May 2017, Grand Hotel Duomo, Pisa
Session 1: Interaction Between ERN and Rare Diseases National Centres
The last day of the kick-off meeting opened with a presentation from Domenica Taruscio (Italian National Centre for Rare Diseases) on INTERACTION BETWEEN ERN and RARE DISEASES NATIONAL CENTRES: THE EXAMPLE OF ITALY.
The Istituto Superiore di Sanità (ISS) and the Italian National Centre for Rare Diseases are organising in Rome the 5th International Summer School on Rare Disease and Orphan Drug Registries from September 18th to September 22nd. During the selection process, priority will be given to members and patients involved in the ERNs. Deadline for application: 30 June 2017. Professor Matthias Schneider will attend the 2017 Summer School on behalf of ReCONNET.
For info: http://www.iss.it/cnmr/?lang=1&id=2739&tipo=3
Session 3: The Patient involvement on ERN ReCONNET
The last session of the meeting was dedicated to Patients Involvement with a presentation on PATIENT INVOLVEMENT IN ERN ReCONNET from Diana Marinello, the Project Manager of ERN ReCONNET. Patients involvement in the Network is one of the key aspects of the project, and therefore the role of patients is strongly integrated in the Governance of ERN ReCONNET.
Patients are involved in all aspects of the Network: they are members of the Steering Committee (one patient for each thematic area) and like the HCP members, they will have to appoint a Disease Coordinator for each disease who will be member of Executive Board and will work with the HCPs disease coordinators.
Patients will also have a dedicated Working Group (Patients Organisation Working Group) that will include a Senior, a Junior Coordinator, all Patient Disease Coordinators, ePAGs Representative and all ePAGs members.
ePAGs representatives will have central role in many activities, including:
- To give the opinion and views of the patient groups;
- Propose methods for feedback and evaluation of patient experience;
- Evaluation of how the ERN acts on feedback from patients;
- Reviewing the performance of the ERN and contributing to research;
- Promote and encourage a patient-centric approach;
- Development and dissemination of patient information;
- Ensure patient rights and choices are taken into account in decision-making;
- Identify relevant national patient organizations to work with the HCPs;
- Identification of expert centres to join the ERN;
- Development a Patients Involvement Terms of Reference.
The four ERN ReCONNET ePAGS introduced themselves:
- Jürgen Grunert from Germany, represents Deutsche Ehlers Danlos Initiative e.V.,
- Ilaria Galetti from Italy, represents FESCA-Federation of European Scleroderma Association and GILS,
- Sara Badreh from the Netherlands, represents Lupus Europe,
- Charissa Frank from Belgium, represents Vlaamse Vereniging voor Erfelijke and Bindweefselaandoeningen vzw.
Patients representatives expressed their enthusiasm and their high expectations on the ERNs that will, in their opinion, change many aspects of patients with rare diseases across Europe by:
- reducing inequalities of treatment amongst different diseases and countries within Europe;
- providing better and harmonized access to medication and treatment in multi disciplinary clinics;
- sharing knowledge, experience, medical research, teaching, training and resources;
- harmonization and development of diagnostic and therapeutic guidelines and cross border pathways;
- promoting and supporting collaborative research amongst Members and Affiliated Partners;
- facilitating the use of eHealth and telemedicine.
HCP members and patient representatives agreed that the real strength of the Network is the strong collaboration with patients, that will work closely with the HCPs as equal partners. A real double win: win for patients and win for clinicians.
Agreement among the participants that the ERNs represent a great opportunity and that ERNs have the potential of changing millions of lives making cross-border care a reality.
Session 4: ERN ReCONNET Future Meetings
The meeting ended with a discussion on future meetings of ERN ReCONNET. The next meeting is scheduled for June 15th, during the EULAR 2017 congress in Madrid. Another proposed meeting is the ACR 2017 meeting, that will take place in San Diego (US) 3-8 November 2017.



